Researchers use CRISPR and stem cells to discover new chlamydia drug targets
May 2, 2017
May 2, 2017

University of British Columbia and Wellcome Trust Sanger Institute researchers have used gene editing techniques on stem cells to identify novel drug targets for chlamydia, the most commonly reported bacterial STI in Canada.
The team identified two immune system genes, IRF5 and IL-10RA, as key players in fighting a chlamydia infection. The results were reported in Nature Communications.
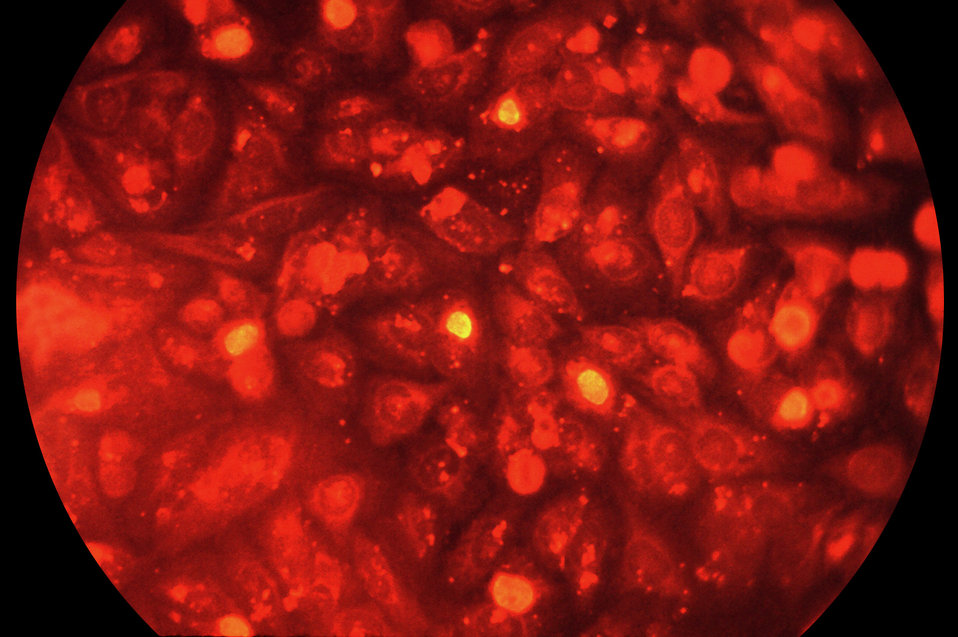
In the study, scientists created white blood cells, called macrophages, from human induced pluripotent stem cells. The lab-produced macrophages responded to chlamydia infection in a similar way to those taken from human blood.
“We can knock out specific genes in stem cells and look at how the gene editing influences the resulting macrophages and their interaction with chlamydia,” says Robert Hancock, lead author from the University of British Columbia and Associate Faculty member at the Wellcome Trust Sanger Institute.
“We’re effectively sieving through the genome to find key players and can now easily see genes that weren’t previously thought to be involved in fighting the infection.”
The new model has advantages over previous methods that used macrophages either derived from mice, which differ from humans in their immune response, or immortalised human macrophage cell lines, which are genetically different to normal macrophages.
“Chlamydia is tricky to study because it can permeate and hide in macrophages where it is difficult to reach with antibiotics,” says Amy Yeung, first author from the Wellcome Trust Sanger Institute.
“Inside the macrophage, one or two chlamydia cells can replicate into hundreds in just a day or two, before bursting out to spread the infection. This new system will allow us to understand how chlamydia can survive and replicate in human macrophages and could have major implications for the development of new drugs.”
The team discovered two macrophage genes in particular that were key to limiting chlamydia infection: IRF5 and IL-10RA. When these genes were switched off, the macrophages were more susceptible to chlamydia infection. The genes could be drug targets for new chlamydia treatments.
Professor Gordon Dougan, senior author from the Wellcome Trust Sanger Institute said: “This system can be extended to study other pathogens and advance our understanding of the interactions between human hosts and infections. We are starting to unravel the role our genetics play in battling infections, such as chlamydia, and these results could go towards designing more effective treatments in the future.”
It is estimated that 131 million people globally are infected with chlamydia each year. Often called the ‘silent disease’, as it rarely produces symptoms early on, chlamydia causes genital infections which can lead to pelvic inflammatory disease and infertility if left untreated.
The increasing threat of antibiotic resistance have led the World Health Organisation to issue new guidelines in 2016 for the treatment of chlamydia. To develop new therapeutics for the infection, its interaction with our immune system must first be understood.
We honour xwməθkwəy̓ əm (Musqueam) on whose ancestral, unceded territory UBC Vancouver is situated. UBC Science is committed to building meaningful relationships with Indigenous peoples so we can advance Reconciliation and ensure traditional ways of knowing enrich our teaching and research.
Learn more: Musqueam First Nation